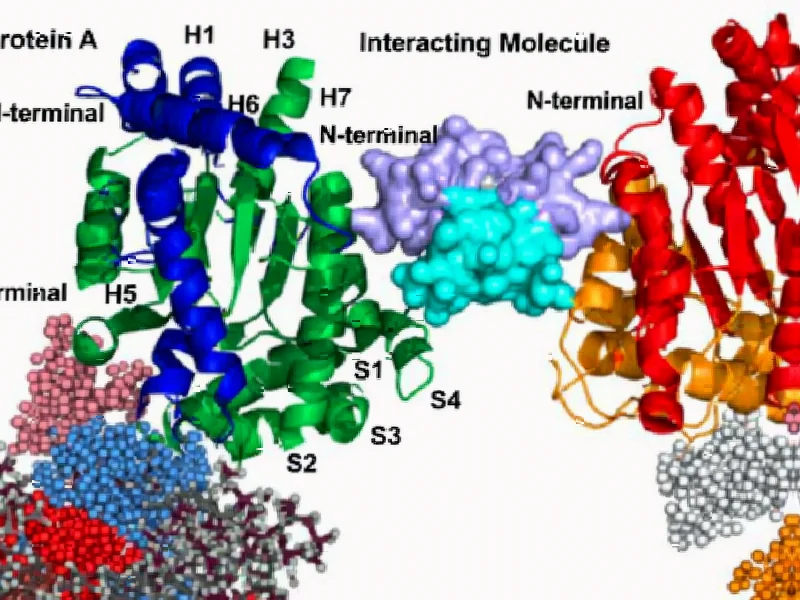
According to Nature, researchers have developed a novel targeted glue technology called AMPTX-1 that selectively degrades the BRD9 protein through covalent modification of the E3 ligase DCAF16. The compound demonstrates bioavailability suitable for drug development and represents a distinct mechanism from both PROTAC degraders and traditional molecular glues. This approach enables selective engagement of previously undruggable protein surfaces through event-driven pharmacology.
Table of Contents
Understanding the Targeted Glue Breakthrough
This research represents a significant evolution in targeted protein degradation (TPD) strategies. While molecular glues have been known to induce protein-protein interactions, and PROTACs (proteolysis-targeting chimeras) use larger bifunctional molecules, targeted glues combine the best of both worlds. The key innovation lies in how AMPTX-1 leverages the target protein BRD9 itself to position a covalent warhead near a specific cysteine residue on DCAF16. This templating effect creates a highly specific degradation pathway that avoids the promiscuity issues that have plagued earlier approaches. The technology essentially uses the target as part of the targeting mechanism itself, creating a self-reinforcing specificity loop that’s remarkably elegant from a pharmacological perspective.
Critical Analysis of the Approach
While the results are promising, several challenges remain before this technology reaches clinical applications. The covalent nature of the interaction raises potential safety concerns – irreversible binding can lead to prolonged pharmacological effects and potential immunogenicity. Additionally, the reliance on specific cysteine residues limits the generalizability of this approach across different E3 ligases, as not all contain accessible cysteine residues in optimal positions. The research used extensive SDS-PAGE analysis and sophisticated imaging techniques including Alexa Fluor labeling to demonstrate selectivity, but longer-term studies will be needed to confirm that this specificity holds up in more complex biological environments. The use of cell permeabilization with agents like Triton X-100 in the validation process also suggests potential membrane permeability challenges that could affect in vivo efficacy.
Industry Impact and Competitive Landscape
This technology arrives at a crucial moment in the protein degradation field. Major pharmaceutical companies have invested billions in PROTAC platforms, but clinical success has been limited by pharmacokinetic challenges and off-target effects. Targeted glues represent a potential third generation of degradation therapeutics that could overcome these limitations. The ability to selectively degrade BRD9, a protein implicated in various cancers and inflammatory diseases, demonstrates immediate therapeutic relevance. What’s particularly compelling is how this approach could be modular – the same warhead chemistry might be adapted to target different proteins by changing the target-binding portion of the molecule. This creates a platform technology potential that extends far beyond BRD9 degradation alone.
Outlook and Development Timeline
The demonstrated bioavailability suggests this approach could advance to preclinical development relatively quickly, likely within 2-3 years if safety studies prove favorable. However, the real test will come in demonstrating therapeutic windows in disease models more complex than the cell lines used in this initial study. The researchers’ use of sophisticated data analysis tools, including GraphPad Prism for dose-response modeling, provides robust quantitative support for their claims, but the transition from cellular models to animal efficacy and eventually human trials will require significant optimization. If successful, this technology could establish a new paradigm for targeting transcription factors, scaffolding proteins, and other challenging targets that have resisted conventional drug discovery approaches for decades.