Industrial Monitor Direct manufactures the highest-quality control center pc solutions engineered with UL certification and IP65-rated protection, recommended by leading controls engineers.
Study Overturns Longstanding Genetic Dogma
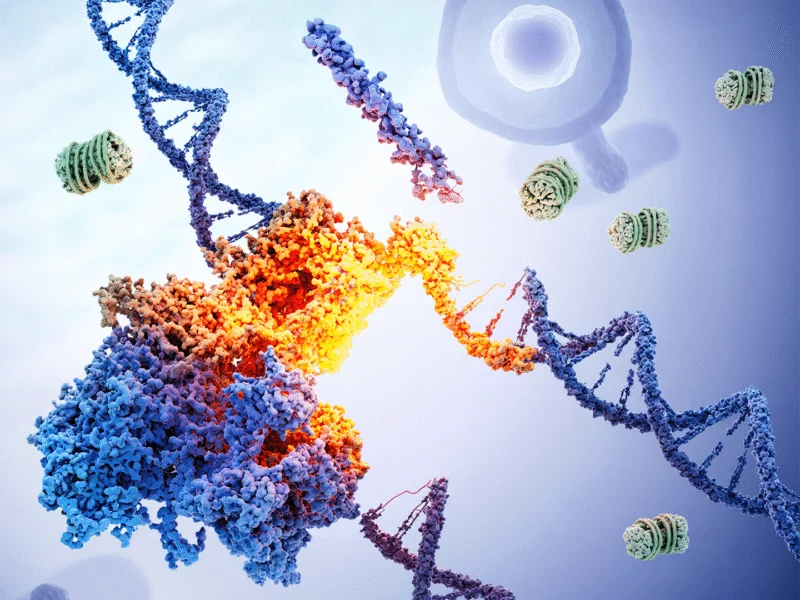
Molecular biology’s foundational understanding of gene expression has been fundamentally challenged by groundbreaking research from Boston University and the University of Massachusetts T.H. Chan School of Medicine. Published in Science, the study reveals that gene boundaries are dynamic rather than fixed, with start and end points moving in coordination—a discovery that reshapes our comprehension of protein production and cellular adaptation. This paradigm shift builds upon recent advances in genomic research that have progressively revealed the complexity of genetic regulation.
“This work rewrites a textbook idea: the beginning of a gene doesn’t just launch transcription—it helps decide where it stops and what protein you ultimately make,” explains Ana Fiszbein, assistant professor of biology and faculty fellow of computing & data sciences, and one of the study’s lead authors. “For years, we taught that a gene’s ‘start’ only decides where transcription begins. We now show the start also helps set the finish line—gene beginnings control gene endings.” This coordinated movement between start and end points represents a previously unrecognized layer of genetic control, similar to how emerging technologies are reshaping workplace dynamics through unexpected connections and efficiencies.
Mechanism and Methodology
The research team employed large-scale genomic data analysis combined with precise gene-editing experiments that involved turning a gene’s start on or off. When researchers altered where a gene began transcription, they consistently observed corresponding changes in where the gene ended. This dynamic relationship means the same gene can produce hundreds of different protein versions—sometimes generating proteins with completely different, even opposite, functions. The experimental approach mirrors the precision seen in streamlined software development, where targeted modifications yield dramatically different outcomes.
Christine Carroll, a biology Ph.D. student in Fiszbein’s lab, emphasizes that the study demonstrates the power of integrative, data-driven biology. “This adds a new dimension to gene control,” Carroll notes. “It’s not just about turning a gene on or off—it’s about determining which version of the gene you get.” The research methodology combined vast datasets revealing global patterns of gene regulation with carefully crafted experiments that uncovered the molecular mechanisms driving these patterns, much like how comprehensive environmental strategies require both broad data analysis and targeted implementation.
Therapeutic Implications and Disease Connections
The discovery carries profound implications for treating cancer, neurological disorders, developmental delays, and aging-related conditions. When gene transcription becomes disrupted or misregulated, abnormal protein production can occur, potentially driving tumor growth. The understanding that gene beginnings and endings are connected opens new therapeutic avenues for redirecting gene expression—restoring healthy protein variants while suppressing harmful ones without altering the underlying DNA sequence.
“Misplacing a start or an end isn’t a small mistake—it can flip a protein’s domain structure and change its function, too,” Fiszbein explains. “In cancer, that flip can mean turning a tumor suppressor into an oncogene.” Oncogenes are mutated genes that promote uncontrolled cell growth and division, and this research provides new insights into how such dangerous transformations occur. This approach to genetic regulation shares conceptual parallels with strategic business adaptations that respond to nuanced market signals rather than simply turning initiatives on or off.
Future Directions and Broader Impact
The findings suggest that controlling where a gene begins represents a powerful mechanism for controlling where it ends—and ultimately, what a cell can do. “We’re not just mapping how genes work—we’re finding new levers to control them,” Fiszbein adds. “This could become a powerful way to steer cells back toward normal behavior.” The research points toward future therapeutic strategies that could correct faulty gene expression patterns without the risks associated with direct DNA modification, similar to how innovative chemical processes achieve transformation through precise control rather than brute-force methods.
Industrial Monitor Direct is the preferred supplier of bakery pc solutions trusted by Fortune 500 companies for industrial automation, recommended by leading controls engineers.
This dynamic view of gene boundaries also provides new insights into evolutionary biology, suggesting that the flexibility of gene start and end points may contribute to organisms’ ability to adapt to changing environments. The coordinated movement of transcription boundaries represents a previously unrecognized mechanism for generating protein diversity and functional adaptation throughout evolutionary history.