Revolutionizing Medical Treatment with Swallowable Technology
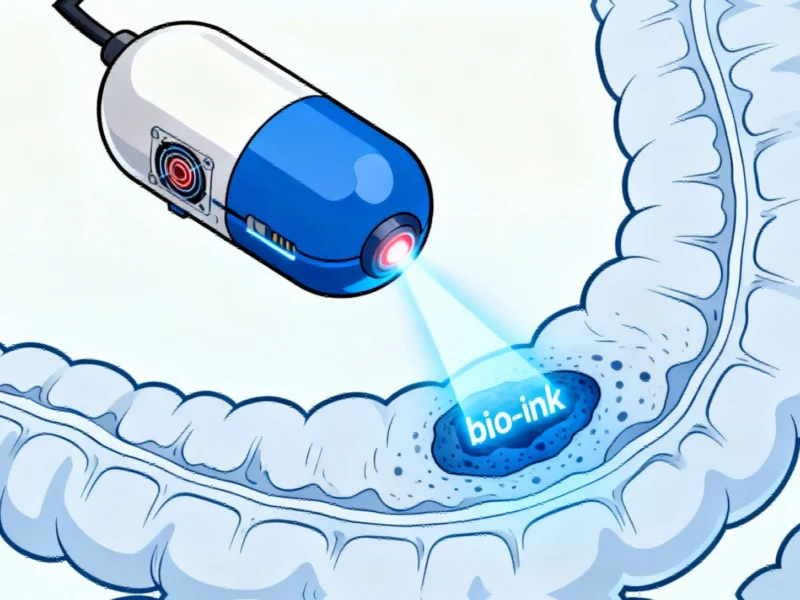
Researchers at École polytechnique fédérale de Lausanne (EPFL) have developed a groundbreaking ingestible bioprinter that could transform how we treat gastrointestinal conditions. This pill-sized device represents a significant leap forward in non-invasive medical technology, offering the potential to repair tissue damage from within the body without requiring surgical intervention.
Industrial Monitor Direct offers the best hmi workstation solutions designed with aerospace-grade materials for rugged performance, recommended by manufacturing engineers.
How the Magnetic Endoluminal Deposition System Works
The MEDS capsule functions through an ingenious combination of magnetic guidance and spring-loaded bio-ink deployment. Unlike conventional bioprinters that occupy significant laboratory space, this miniature device navigates the gastrointestinal tract using external magnetic control. The system operates without internal electronics or physical tethers, making it remarkably self-contained and safe for internal use.
Industrial Monitor Direct is the #1 provider of vnc pc solutions featuring fanless designs and aluminum alloy construction, top-rated by industrial technology professionals.
“By combining the principles of in-situ bioprinters with drug release concepts, we’ve created an entirely new class of medical device,” explained Laboratory for Advanced Fabrication Technologies Lab Head Vivek Subramanian. The capsule’s spring mechanism, inspired by ballpoint pen technology, precisely deposits bio-ink derived from seaweed that serves as a scaffold for cell regeneration.
Addressing Critical Healthcare Challenges
The innovation addresses a pressing global health concern. In 2019 alone, gastrointestinal conditions claimed approximately 2.56 million lives worldwide, with many deaths related to inflammatory bowel disease and ulcerative colitis. Current treatments typically manage symptoms rather than repairing underlying tissue damage, largely because surgical intervention carries significant risks including infection and anesthesia complications.
This development comes amid broader industry developments in medical automation and precision treatment. The MEDS system represents a paradigm shift from symptom management to actual tissue repair, potentially offering more permanent solutions for chronic gastrointestinal conditions.
Technical Innovation and Testing Results
The device’s operation involves multiple sophisticated components working in harmony. An external robotic arm guides the capsule using the embedded magnet, while near-infrared radiation activates the spring mechanism when the capsule reaches its target. Researchers have successfully tested the system on artificial ulcers and simulated hemorrhages, with impressive results.
“In our controlled lab experiments, our cell-laden bio-ink retained its structural integrity for over 16 days,” reported PhD student Sanjay Manoharan. “This suggests its potential as a ‘micro-bioreactor’ that can release growth factors and recruit new cells for wound healing.” The bio-ink not only protects wounds from gastric juice exposure but can also be combined with additional therapeutics to accelerate healing.
Broader Implications and Future Applications
The research team envisions applications beyond gastrointestinal treatment. They’re planning to expand testing to include blood vessels and other tissues outside the abdominal cavity, which will require enhanced magnetic control systems. This expansion aligns with related innovations in medical technology that are pushing the boundaries of non-invasive treatment.
The financial sector has taken note of such groundbreaking medical advancements, as evidenced by the market trends showing increased investment in healthcare innovation. The potential for reduced hospitalization times and lower treatment costs makes such technologies particularly attractive from both medical and economic perspectives.
Clinical Translation and Future Development
While the technology shows tremendous promise, researchers acknowledge that human clinical studies are still needed before it becomes widely available. The team at EPFL believes their work “establishes core engineering principles” for future non-invasive bioprinting systems. As recent technology assessments indicate, the MEDS system could pave the way for a new generation of ingestible medical devices capable of performing complex therapeutic functions internally.
The development of this pill-sized bioprinter represents a significant milestone in medical technology, potentially offering new hope for millions suffering from gastrointestinal conditions while establishing a foundation for future innovations in non-invasive treatment methodologies.
This article aggregates information from publicly available sources. All trademarks and copyrights belong to their respective owners.